Understanding ECMO Physiology
- Tyer Christifulli
- Jul 9, 2021
- 5 min read

The concept of taking blood out of the body, oxygenating it, removing the CO2, and then putting back in, fascinates me. A few years ago I admittedly knew very little about extracorporeal membrane oxygenation (ECMO) and its indications. I remember going to a class on ECMO at Life Link III and having questions like:
Are we actually pumping blood backwards through the body?
What happens to the blood in the heart when using ECMO in cardiac arrest (ECPR)?
What kind of vent settings should I use?
I am by no means an expert on ECMO, in fact, I have only been on a handful of ECMO transports, but the concept fascinates me and I thought a blog breaking down a few concepts of ECMO physiology would be beneficial.
Are we actually pumping blood backwards through the body?
The two most common types of ECMO configurations are V-A and V-V. The difference between the two is where you return the oxygenated blood. In a V-V setup, the blood is being taken from the vein, oxygenated, and put back into a vein. You can imagine why V-V requires adequate cardiac output. Circulating that oxygenated blood requires a functioning heart.

In VA ECMO we are taking blood from the vein (pretty much right next to the right atrium), oxygenating it, and then putting it back into the artery. As blood is pumped into the arterial tree it is pressurized. This allows restoration of perfusion to end organs as demonstrated below.

By pressurizing the arterial tree with oxygen-rich blood, we can hopefully increase coronary perfusion pressure enough to restore adequate cardiac function. If heart function returns, it's important to avoid a high afterload that could compromise forward flow. This is one reason why pressor therapy can typically be reduced or stopped after a patient is put on ECMO.
Can pumping blood into the aorta cause congestive heart failure?
I use to imagine a sick left ventricle trying to contract against a machine pumping blood into the aorta at 3-5 lpm. If the MAP is increased beyond the minimal opening pressure of the left ventricle, wouldn't this prevent forward flow? Actually, yes. Supraphysiologic after loads from retrograde flow can cause pulmonary edema, mitral valve prolapse, amongst other complications (1). While you don't want your MAP to be so low that you aren't adequately perfusing vital organs, a high MAP can cause just as many complications (3). There really is no magic number here when it comes to a goal MAP. The goal should be to restore native forward flow, and when that is not possible without drastically decreasing MAP, we need a ventricular assist device (i.e Impella, TandemHeart, IABP, etc). Pictured below is an Impella device. This will suck blood from the LV and pump it into the aortic arch.
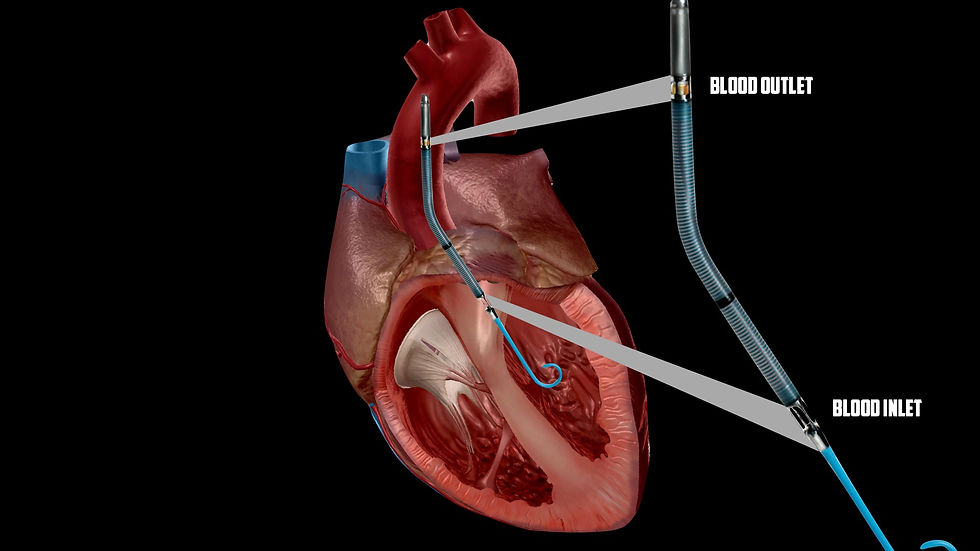
Perhaps LV function is able to keep forward flow, but the EF is poor. To optimize coronary perfusion, it helps to not have a bunch of blood remaining in the left ventricle pushing against the walls in which the coronary flow must overcome. An LV drain can be placed to reduce left ventricular end-diastolic pressure enough to stay on the optimal side of the Frank-Starling curve. You can imagine that if too much blood is drained from the LV, cardiac output will diminish. The LV drain will connect to the venous limb of the ECMO circuit (1).

What kind of vent settings should I use?
The term most often used in terms of vent strategy during ECLS is "rest" settings. This can be subjective and depend on your shop, but it likely means a lung-protective strategy including low tidal volumes and minimal driving pressure. In an international survey on ventilator settings during ECMO, 76% of ECMO centers reported using 6mL/kg or less (2). It is important to point out the value of looking at volume over pressure. I sometimes hear clinicians say "I don't care about the volumes as long as my pressures are within a safe range." In reality, both are important data points - but ventilating with excessive volumes because your pressures are within a safe range could lead to over distention. There is an excellent article in the Canadian Respiratory Journal from 2017 that I included below. (4).

Intrapulmonary Shunt
As native heart function improves, it is important to optimize ventilator settings in order to not create a hypoxic intrapulmonary shunt (4). When poorly oxygenated blood is pumped through the heart and into the aorta, a mixing cloud occurs. This is when the native blood flow meets the ECMO flow and competes for room within the aorta. Unfortunately, this cloud commonly occurs within the aortic arch as illustrated below.

You can imagine with this location of the mixing cloud, the coronary arteries are first to suffer. This is one reason why every ECMO patient with have a right radial arterial line placed. The right radial arterial blood gas is the closest guess as to what blood the coronary arteries and brain are seeing.
The first fix when a mixing cloud is present is to try and optimize your ventilator settings. If that does not work, a branch of the arterial limb can be added and connected to the venous circuit. This is referred to as V-A-V ECMO.
I hope you found this quick-hit Q&A helpful, and I would love to hear what questions you have about ECMO. Email us your questions at team@foamfrat.com. If you are interested in learning more about ECMO I encourage you to check out the FOAMfrat EMS/Nursing library which includes live classes five days a week. I and several others have put together talks outlining ECMO physiology, ECPR, and pharmacology.
PEER REVIEW:
Brian King
Now more than ever is ECMO becoming more common outside of the cardiac OR and cardiac ICUs. You are seeing it being deployed in a number of emergency departments and even the prehospital setting making it that much more important to understand the fundamental concepts of ECMO. By building upon the basic concepts and physiology of ECMO you will gain a much better understanding of how and why ECMO works.
References:
1. Cevasco, M., Takayama, H., Ando, M., Garan, A., Naka, Y., & Takeda, K. (2019). Left ventricular distension and venting strategies for patients on venoarterial extracorporeal membrane oxygenation. Journal Of Thoracic Disease, 11(4), 1676-1683. doi:10.21037/jtd.2019.03.29
2. Marhong J. D., Telesnicki T., Munshi L., Del Sorbo L., Detsky M., Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Annals of the American Thoracic Society. 2014;11(6):956–961. doi: 10.1513/AnnalsATS.201403-100BC.
3. Schmack, Bastian & Seppelt, Philipp & Weymann, Alexander & Alt, Christina & Farag, Mina & Arif, Rawa & Doesch, Andreas & Raake, Philip & Kallenbach, Klaus & Mansur, Ashham & Popov, Aron & Karck, Matthias & Ruhparwar, Arjang. (2017). Extracorporeal life support with left ventricular decompression-improved survival in severe cardiogenic shock: Results from a retrospective study. PeerJ. 5. e3813. 10.7717/peerj.3813.
4. Zhang, Z., Gu, W. J., Chen, K., & Ni, H. (2017). Mechanical Ventilation during Extracorporeal Membrane Oxygenation in Patients with Acute Severe Respiratory Failure. Canadian respiratory journal, 2017, 1783857. https://doi.org/10.1155/2017/1783857





