Ventilation - Playing Defense (Part 1)
- Tyer Christifulli
- Mar 21, 2021
- 5 min read

In any game there are offensive and defensive strategies. Generally speaking, defensive strategies prevent you from losing, and offensive maneuvers get you points. When I think of mechanical ventilation in general, we are always playing defense. The goal with mechanical ventilation is to not kill the patient while they recover. Liberation from the ventilator is always the ultimate goal.
In this blog I want to address mechanical ventilation in the patient with a metabolic acidosis. "Intubation" and "mechanical ventilation" in the same sentence should give you shivers down your spine. We know that mechanical ventilation does not fix a metabolic acidosis. The "fixing" of a metabolic acidosis would be an offense move, and is focused on identifying the underlying cause.

Occasionally a patient with metabolic acidosis will have a deterioration in mental status and require airway protection and thus intubation. How you ventilate this patient will drastically increase or decrease their chance of survival.
If the body can not fix a metabolic acidosis with ventilation, why does minute volume nearly double during severe metabolic acidosis?

pH is an indicator, a thermostat. If my thermostat says my house dropped by 10 degrees, this could mean:
Someone left a window open.
Someone left a door open.
My heater is broke
The point being, we shouldn't get tunnel visioned on the pH. Rather, we should try to figure out why the thermostat is indicating a change in homeostasis.
Let's pretend we have a patient that presents with diabetic ketoacidosis. Because their cells can not utilize sugar, they will burn fat and produce ketones. Ketones are a weak acid and will be stacked on the anion or negatively charged column. To maintain electroneutrality, the body will get rid of bicarbonate in order to make room for these ketones (Morgan, 2009). The more ketones, the less bicarbonate.
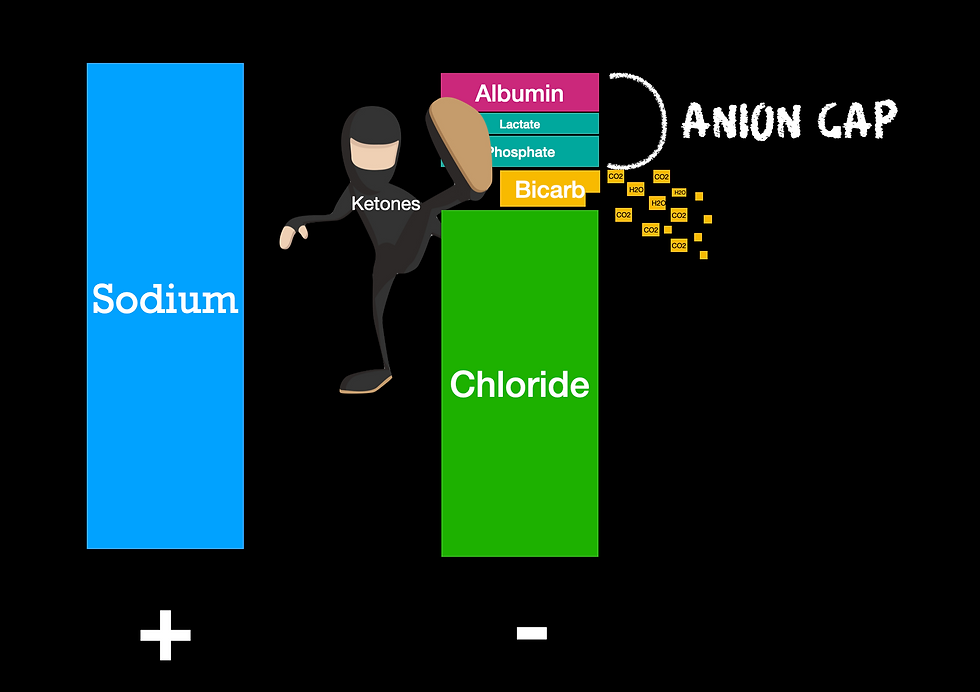
That bicarbonate will be disassociated into CO2 and H2O. If you look at the picture above, you will see that CO2 is indeed one of the independent variables to pH. This means that if we do not blow off the CO2, we will not only have an addition of weak acids decreasing our pH, but now an accumulation of carbon dioxide. That means we have 2/3 independent variables working against our pH. Throw on 0.9% sodium chloride and you will have the trifecta.
The Diagnosis
The first step in assuring you are properly ventilating a patient in metabolic acidosis, is to know/suspect that they are in a metabolic acidosis. Pretend you walk into the ED and I hand you the following ABG.
pH 7.11
PCO2 14
PO2 200
HCO3 7
If you concluded that this patient is in a partially compensated metabolic acidosis, you are correct. While only one of those parameters is an independent variable of pH (CO2), this definitely puts "metabolic acidosis" on your radar. Now what do we do with that that information in transport?
Step 1. Determine your ETCO2 and PaCO2 gradient. Your PaCO2 is the source and the ETCO2 is the byproduct. This means your ETCO2 will ALWAYS be lower than your PaCO2. Depending on perfusion and health of the alveoli, this range can be extremely wide. You may see an ETCO2 of 35 and a PaCO2 of 70. Can you see why it would be dangerous to titrate minute volume to your ETCO2 w/o knowing the gradient?
Step 2A. Theoretically you can calculate something called a 'Winter's Correction" to determine your max respiratory compensation for a given bicarb (Morris, 2020). The formula is pretty simple:

So let's say we have a patient with a bicarb level of 18 and want to know what max respiratory compensation would look like in regards to PaCO2. We would work the equation as:

So the reality is, I don't think anyone is actually calculating this junk on the fly. Where I work we do not routinely draw ABG's - which is fine because the bicarb on an ABG actually is calculated and not measured. If you want the true bicarb, you need to look at the CO2 on the chem panel, and we can get this on an EPOC.
So can we use Winter's correction to target a PvCO2? It depends. Depending on the metabolic demands of the body, the PaCO2 and PvCO2 can have a wide discrepancy. The best way to tell if the two correlate, is to look at the SVO2 (venous saturation). Typically blood should return to the heart 75% saturated with oxygen. This means the body only used 25% of the oxygen delivered. If the blood is returning with a saturation < 75%, it is likely your PaCO2 to PvCO2 gradient is wide (Lemoël, 2012) . Your decision tree may look something like this.

Step 2B. Plan B assumes that it is very unlikely you are going to hyperventilate your patient in a metabolic acidosis. The goal is to increase the minute volume as much as you can without causing air trapping. If your ventilator has a flow waveform graphic, you will utilize the "no-flow" zone to optimize inspiratory time or rate as seen below.

In a patient with healthy lungs, increasing tidal volume is a more efficient way to increase alveolar minute volume as compared to just increasing rate. Each breath comes with a specific amount of dead space and if you can get more alveolar volume for the same amount of dead space, you are optimizing ventilation.
If you do not have waveform graphics on your ventilator you will need to watch for air trapping by monitoring plateau pressures and exhaled tidal volume. You can also check for auto peep by performing an exhalation hold.
Take Home Points
1. It is very difficult to meet the minute volume of a spontaneous breathing patient in metabolic acidosis with a mechanical ventilator. For this reason, intubation should be avoided at all costs.
2. If mental status deteriorates to the point of intubation, it will be very difficult to cause harm by means of hyperventilation if you are allowing flow to return to baseline after each breath.
3. VBG may correlate with ABG if SvO2 is >75%. When labs are not available, gestalt, breathing pattern, and past medical history will be key in identification.
4. Never titrate down a respiratory rate to normalize ETCO2 until you know the PCO2 to ETCO2 gradient.
References:
Lemoël F, Govciyan S, El Omri M, Marquette CH, Levraut J. Improving the validity of peripheral venous blood gas analysis as an estimate of arterial blood gas by correcting the venous values with SvO₂. J Emerg Med. 2013 Mar;44(3):709-16. doi: 10.1016/j.jemermed.2012.07.041. Epub 2012 Aug 24. PMID: 22921853.
Morgan T. J. (2009). The Stewart approach--one clinician's perspective. The Clinical biochemist. Reviews, 30(2), 41–54.
Morris S. Albert, Ralph B. Dell, Robert W. Winters. Quantitative Displacement of Acid-Base Equilibrium in Metabolic Acidosis. Ann Intern Med.1967;66:312-322. [Epub ahead of print 13 March 2020].doi:10.7326/0003-4819-66-2-312




