Christmas in July
- Sam Ireland
- Jul 25, 2025
- 10 min read

Expectation vs. Reality
It’s July 4th, and you’re working a 24. The plan is simple: between calls, stop by the grocery store for grilling supplies for the station cookout, and arrive at the park in your response area by 2130 for your town’s blowout 50th anniversary annual fireworks display.
However, the weather doesn’t agree. It’s an unusually mild July 4th, and the forecast calls for rain and wind. Lows will dip to 62ºF, with a few inches of rain and winds gusting at 25 mph. In short, the fireworks are canceled, and you pick up Chinese instead of grilling out. The obligatory movie “Independence Day” is playing on TV as you hammer chow mein.
No One Told Kelvin
Meanwhile, at the local nursing home, no one informed Kelvin (our future patient) that the fireworks were canceled. Kelvin is 82 years old and has been attending the town’s July 4th fireworks festival every year since its inception. Of note, Kelvin also suffers from Alzheimer’s and dementia, but he still remembers his way to the park after living locally for practically his whole life.
A little more about Kelvin’s medical history:
Sarcopenia: Frail elderly man with little muscle and fat (little insulation).
Diuretics: Takes Lasix but often forgets to take fluids (he doesn’t eat much either - both of these contribute to low energy stores and vascular volume).
Beta Blockers: Takes metoprolol, which lowers his resting heart rate and overall cardiac output (reducing his ability to thermoregulate optimally).
Peripheral Vascular Disease: Has poor limb perfusion (already chronically cold limbs).
Kelvin exits the front door of the nursing home undetected and makes the 0.25-mile trip to the park without incident. He finds a bench and claims his spot. He is undeterred by the rain and wind, convinced the show will start any minute. Compared to his 80ºF room at the nursing home, it’s rather cold outside, and he begins to shiver. The time is now 2200, and his core temperature approaches 95°F (35°C).
In addition to the medical history noted above, Kelvin has a few other age-related issues. As one gets older, several limitations set in:
Blunted autonomic response: Slower vasoconstriction and weaker shiver threshold, so heat-loss pathways stay open longer (Greaney et al., 2015).
Diminished shivering efficiency: Fewer fast-twitch fibers and depleted glycogen result in shivering generating less heat and exhausting fuel quickly (Yang & Chan, 2022).
Cognitive impairment: Delayed behavioral responses, such as not seeking shelter, adding layers, or recognizing early chill.
All of these age-related issues, combined with Kelvin’s other medical history, make him susceptible to hypothermia even in conditions where they wouldn’t usually be expected (such as on the 4th of July).
Heat Loss Mechanisms
Conduction, Convection, Evaporation, and Radiation are all working against Kelvin.
Conduction: His thighs and back press against a metal bench that conducts heat much faster than something like dry wood (Geng et al., 2006). Body warmth flows directly into the cooler metal until the contact areas approach ambient temperature. Wet clothing further boosts conductive transfer.
Convection: 25 mph gusts sweep away the thin layer of air his body has just warmed, replacing it with cooler ambient air over and over.
Evaporation: Cold rain soaks his clothes. Every gram of water that evaporates off the fabric (or drips, leaving fresh droplets to evaporate) steals ~540 cal of heat (Sessler et al., 2008). Wind accelerates this water phase-change loss.
Radiation: At night, he radiates infrared energy toward the colder sky and surrounding surfaces. Since the sun isn’t out, there’s no solar gain to offset the loss.
There are also respiratory issues to consider, as with each breath, he warms and humidifies the cooler ambient air before exhaling it.
Into the Night
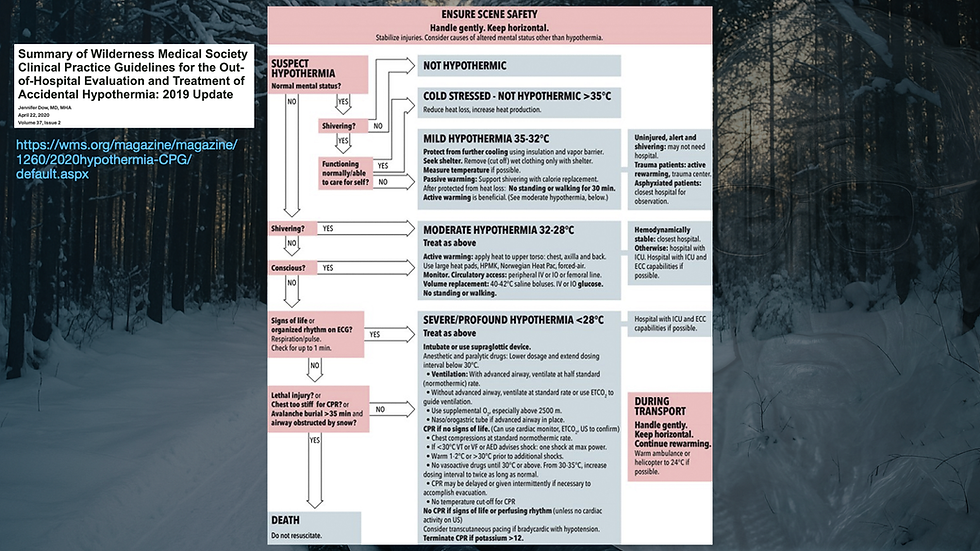
The Swiss Grading System for Hypothermia (Musi et al., 2021) is as follows:
HT-I (Mild): 95 - 89.6°F (35 - 32°C) (conscious and shivering).
HT-II (Moderate): 89.6 - 82.4°F (32 - 28°C) (altered mental status and not shivering)
HT-III (Severe): 82.4 - 75.2°F (24 - 28°C) (unconscious with vital signs)
HT-IV (Profound/Apparent Death): < 75.2°F (< 24°C) (unconscious with no detectible vital signs)
HT-V (DOA): < 48.2°F (< 10°C)

At 2300, Kevlin is in HT-I (conscious and shivering), with a core temperature of ~92°F (32.3°C). He wanders around the park after leaving the cold metal bench, but only manages to become lost and more challenging to find. He slips and trips into a puddle, where he will lie until someone finds him.
At 0001, July 5th, Kelvin stopped shivering, and his core temperature dips to 86°F (30°C), placing him inside the HT-II range (AMS and not shivering). He is now even more altered than his baseline, and his body is no longer attempting to warm itself.
At 0030, an aide at the nursing home notices that Kelvin never got up to use the bathroom and watch TV (this is a typical nightly occurrence). A check of the entire nursing home proves him missing. A Silver Alert is called and a search is underway for a missing 82-year-old man with cognitive impairment, last seen 2000 on July 4th.
At 0100, Kelvin is found by law enforcement in his puddle at the park. He feels cold to the touch, appears pale, still, and exhibits a significantly altered behavior.
At 0115, you arrive on the scene. Law enforcement provides you with his medical history obtained from the aide at the nursing home, which includes beta blockers and diuretic prescriptions, Alzheimer’s and dementia, peripheral vascular disease, and a note about poor oral intake. Upon assessment, you indeed find a confused, cold-limbed, sarcopenic, bradycardic patient that seems to fit the picture his history paints.
At 0130, the patient is now in the back of your ambulance. You notice that his hands feel very cold, and the SPO2 is not providing a pleth wave. His upper arm is seemingly ice cold as you place the blood pressure cuff. Removing his wet house shoes reveals the palest feet you’ve ever seen. You and your partner decide to warm his arms and legs with chemical heat packs, and roll his pants and sleeves up to wrap dry blankets around the extremities. The patient is encouraged to move his limbs to get the blood moving, which he attempts, and the heat in the ambulance is turned up to the maximum.
At 0145, the patient becomes severely bradycardic and hypotensive, and is now unresponsive. Law enforcement notices this unresponsiveness first and gives Kelvin’s shoulders a stern shake - “KELVIN!! Can you hear us?!” The artifact on the ECG seems never to subside after the jostle, and you realize the patient is now in ventricular fibrillation.
What Happened?
We know (now) that Kelvin was at least in HT-II, but he was perhaps bordering HT-III (severe). For the sake of the scenario, let’s say his core temperature was 89°F (31.6°C). At this stage, it’s essential to note a few key points regarding his status - as things get cold, they get slow.
His shivering response is gone. Muscles are rigid, reflexes are slowed, glycogen is depleted, and so is ATP. Temperature drops precipitously after shivering gives way.
His cardiac conduction is slow, but vulnerable. You may observe bradycardia and Osborn Waves (a sign that the epicardium and endocardium have a conduction difference due to temperature) (Cadogan & Buttner, 2022; Omar, 2015). However, it may not stay this way. The heart is also extremely irritable, possibly due to different depolarization and repolarization rates, and jostling the patient may lead to mechanical triggering of ventricular fibrillation. The mechanism behind ventricular irritability in response to movement remains unsettled. Regardless of the exact mechanism, these patients should be handled as if they’re made of ice.
His altered mental status is not only due to his baseline, but there is a decreased cerebral metabolic rate due to temperature/decreased neurotransmitter release (as seen in TTM) (Mori et al., 2012, as cited in Liu & Zhou, 2023). Kelvin also has a high likelihood of being hypoglycemic due to exhausting his energy stores.
He’s prone to bleeding (hypothermia being one of the deadly contributing factors of trauma).
And now for the most interesting part of the scenario…
Afterdrop and 'After Shock' (‘Rewarming shock’)
As the body cools, it redirects blood to the core to maintain the warmth of vital organs. It accomplishes this by vasoconstriction in the skin and extremities, especially. This leads to very cold extremities and a high blood volume core. Why does this compensatory mechanism matter?
First, Afterdrop. Afterdrop occurs when the colder blood from the extremities starts to recirculate back to the core (and the blood from the core flows through the extremities). The arms and legs then become like a blood cooler, and drop the average temperature of the blood throughout the entire body. This is where the name ‘afterdrop’ comes from - it’s a drop in core temperature after you begin warming the patient (Dow et al., 2019; Brown et al., 2015).
Second, After Shock (perhaps ‘cold diuresis-induced hypovolemia’, or ‘rewarming shock’, or ‘circumrescue collapse’). As blood is shunted to the core, the kidneys recognize this increased blood volume and perform a function called cold diuresis (Duong & Patel, 2024). Essentially:
Blood shunts to the core ->
This triggers the kidneys to perceive it as volume overload ->
The hypothalamus responds by decreasing Anti-Diuretic Hormone ->
The patient starts diuresing large amounts of fluid and electrolytes ->
They become hypovolemic.
However, due to the profound vasoconstriction in the extremities and skin, this hypovolemia may remain undetected until the limbs warm and the entire blood volume is redistributed evenly throughout the body. Hence the name 'after shock' - it is hypovolemic shock that is realized after you start rewarming the patient (perhaps akin to removing a septic patient from their phenylephrine infusion). This is at least part of the mechanism described by Bjertnæs et al. (2022). It's likely that some mechanisms of hypotension after rewarming remain unknown.

Both Afterdrop and 'After Shock' occur at least in part due to the same mechanism: opening up circulation and redistributing blood to the cold skin and extremities.
To avoid these complications, it’s essential first to recognize hypothermia and then to focus on rewarming the core before the limbs (Dow et al., 2019). For Kelvin, this would have meant:
Removing wet clothes and wrapping warm blankets around his core, perhaps wrapping the limbs separately.
Placing heat packs wrapped in towels on the axilla, groin, and near the neck.
Active internal rewarming with 104°F (40°C) warmed fluids (for warming and avoiding After-Shock).
Ensure the ambulance is heated to a minimum of 82°F (27.8°C).
Wrapping the core in an emergency blanket.
Oh, and checking the BGL. Kelvin's BGL was 45 mg/dL after burning through so much fuel, and replacing fuel is key to enabling the body to warm up again.
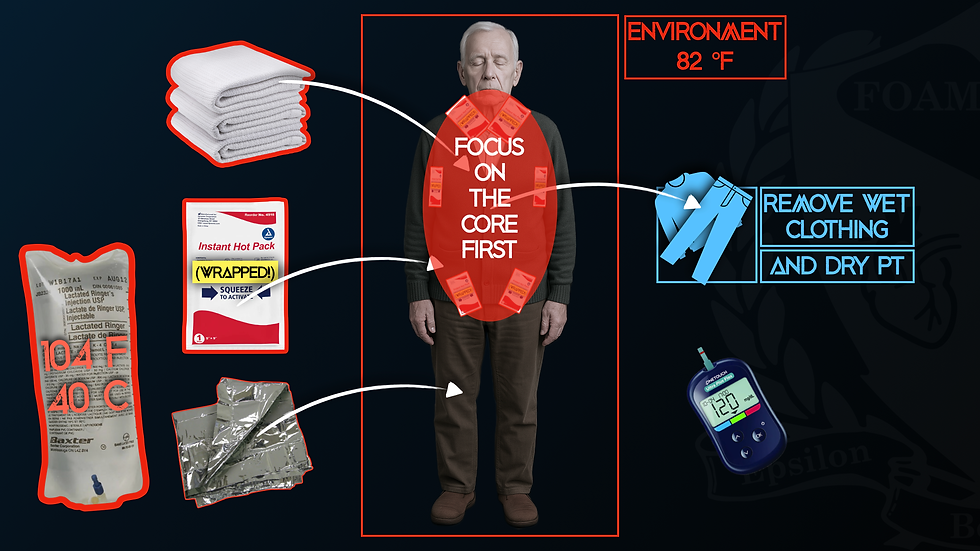
In general, focus on the core, and let the limbs warm up over time. The patient should also be kept supine and not allowed to walk or encouraged to use their limbs (activities such as hand wringing). These recommendations likely do not apply to the mild hypothermic patient (HT-I cases) (Brown et al., 2015). If you have the ability, and it seems indicated (AMS), check a rectal core temperature when in doubt (other methods will likely show erroneous readings).
We didn’t go into what happens after Kelvin loses his pulse, but keep in mind hypothermia guidelines for cardiac arrest. The tempo of electrical and chemical interventions slows down, and the tempo of getting the patient to an ECMO/CPB/ECLS-capable center for rewarming increases (Takauji et al., 2023).
Equilibrium
Even though it wasn’t exactly Christmas, Kelvin’s body was in a process of equilibrium with his environment, becoming the ambient temperature. As the body runs out of energy and loses its compensatory mechanism of shivering, hypothermia may occur even in temperatures where it would not typically be on your list of high-probability differentials.
Thanks for reading!
References
Bjertnæs, L. J., Næsheim, T. O., Reierth, E., Suborov, E. V., Kirov, M. Y., Lebedinskii, K. M., & Tveita, T. (2022). Physiological changes in subjects exposed to accidental hypothermia: An update. Frontiers in Medicine, 9. https://doi.org/10.3389/fmed.2022.824395
Brown, D., Ellerton, J., Paal, P., & Boyd, J. (2015). Hypothermia evidence, afterdrop, and practical experience. Wilderness & Environmental Medicine, 26(3), 437–439. https://doi.org/10.1016/j.wem.2015.01.008
Cadogan, M., & Buttner, R. (2022, February 10). Osborn wave (J wave) overview. Life in the Fast Lane • LITFL. https://litfl.com/osborn-wave-j-wave-ecg-library/
Duong, H., & Patel, G. (2024, January 19). Hypothermia. In StatPearls [Internet]. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK545239
Dow, J., Giesbrecht, G. G., Danzl, D. F., Brugger, H., Sagalyn, E. B., Walpoth, B., Auerbach, P. S., McIntosh, S. E., Némethy, M., McDevitt, M., Schoene, R. B., Rodway, G. W., Hackett, P. H., Zafren, K., Bennett, B. L., & Grissom, C. K. (2019). Wilderness medical society clinical practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia: 2019 update. Wilderness & Environmental Medicine, 30(4_suppl), S47–S69. https://doi.org/10.1016/j.wem.2019.10.002
Falla, M., Micarelli, A., Hüfner, K., & Strapazzon, G. (2021). The effect of cold exposure on cognitive performance in healthy adults: A systematic review. International Journal of Environmental Research and Public Health, 18(18), 9725. https://doi.org/10.3390/ijerph18189725
Geng, Q., Holmér, I., Hartog, D. E., Havenith, G., Jay, O., Malchaire, J., Piette, A., Rintamäki, H., & Rissanen, S. (2006). Temperature limit values for touching cold surfaces with the fingertip. The Annals of Occupational Hygiene, 50(8), 851–862. https://doi.org/10.1093/annhyg/mel030
Greaney, J. L., Alexander, L. M., & Kenney, W. (2015). Sympathetic control of reflex cutaneous vasoconstriction in human aging. Journal of Applied Physiology, 119(7), 771–782. https://doi.org/10.1152/japplphysiol.00527.2015
Hewett Brumberg, E. K., Douma, M. J., Alibertis, K., Charlton, N. P., Goldman, M. P., Harper-Kirksey, K., Hawkins, S. C., Hoover, A. V., Kule, A., Leichtle, S., McClure, S., Wang, G., Whelchel, M., White, L., & Lavonas, E. J. (2024). 2024 american heart association and american red cross guidelines for first aid. Circulation, 150(24). https://doi.org/10.1161/cir.0000000000001281
Liu, H., & Zhou, M. (2023). The utility of therapeutic hypothermia on cerebral autoregulation. Journal of Intensive Medicine, 3(1), 27–37. https://doi.org/10.1016/j.jointm.2022.08.004
Lott, C., Truhlář, A., Alfonzo, A., Barelli, A., González-Salvado, V., Hinkelbein, J., Nolan, J. P., Paal, P., Perkins, G. D., Thies, K.-C., Yeung, J., Zideman, D. A., Soar, J., Khalifa, G., Álvarez, E., Barelli, R., Bierens, J. J., Boettiger, B., Brattebø, G.,...Schmitz, J. (2021). European resuscitation council guidelines 2021: Cardiac arrest in special circumstances. Resuscitation, 161, 152–219. https://doi.org/10.1016/j.resuscitation.2021.02.011
Mori, K., Maeda, M., Miyazaki, M., & Iwase, H. (2012). Effects of mild and moderate hypothermia on cerebral metabolism and glutamate in an experimental head injury. In Intracranial pressure and neuromonitoring in brain injury (pp. 222–224). Springer Vienna. https://doi.org/10.1007/978-3-7091-6475-4_64
Musi, M. E., Sheets, A., Zafren, K., Brugger, H., Paal, P., Hölzl, N., & Pasquier, M. (2021). Clinical staging of accidental hypothermia: The revised swiss system. Resuscitation, 162, 182–187. https://doi.org/10.1016/j.resuscitation.2021.02.038
Mydske, S., & Thomassen, Ø. (2020). Is prehospital use of active external warming dangerous for patients with accidental hypothermia: A systematic review. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine, 28(1). https://doi.org/10.1186/s13049-020-00773-2
National Association of State EMS Officials. (2022, March). National model EMS clinical guidelines (Version 3.0). https://ems.utah.gov/wp-content/uploads/sites/34/2024/05/National-Model-EMS-Clinical-Guidelines_2022.pdf
Omar, H. R. (2015). The osborn wave: What have we learned? Herz, 41(1), 48–56. https://doi.org/10.1007/s00059-015-4338-8
Sessler, D., Warner, D., & Warner, M. (2008). Temperature monitoring and perioperative thermoregulation. Anesthesiology, 109(2), 318–338. https://doi.org/10.1097/aln.0b013e31817f6d76
Takauji, S., Hayakawa, M., Yamada, D., Tian, T., Minowa, K., Inoue, A., Fujimoto, Y., Isokawa, S., Miura, N., Endo, T., Irie, J., Otomo, G., Sato, H., Bando, K., Suzuki, T., Toyohara, T., Tomita, A., Iwahara, M., Murata, S.,...Sato, Y. (2023). Outcome of extracorporeal membrane oxygenation use in severe accidental hypothermia with cardiac arrest and circulatory instability: A multicentre, prospective, observational study in japan (ice-crash study). Resuscitation, 182, 109663. https://doi.org/10.1016/j.resuscitation.2022.12.001
Tveita, T., & Sieck, G. C. (2022). Physiological impact of hypothermia: The good, the bad, and the ugly. Physiology, 37(2), 69–87. https://doi.org/10.1152/physiol.00025.2021
Yang, Q., & Chan, P. (2022). Skeletal muscle metabolic alternation develops sarcopenia. Aging and disease, 13(3), 801. https://doi.org/10.14336/ad.2021.1107




